蒸汽灭菌中的最大装载和最小装载发表时间:2024-08-02 09:16
Steam sterilization is a critical process in the manufacture of many pharmaceutical and medical device products. Because of its importance and wide usage it receives a great deal of attention from both practitioners and regulators. Despite this focus, there are aspects relating to load size that prove troublesome, and can cause difficulty during sterilization cycle development, and validation. Regulators will routinely query users on their means for validation of varying load sizes in both pre-approval and routing inspection. The regulatory expectation is that the user has validated fixed and invariable load patterns, and thus load sizes for all sterilization processes. This is expected for both parts (porous) and terminal (non-porous) loads. While defined loads has been used by some firms, their use constrains operational flexibility and many industrial users have sought more flexible, and efficient means. Depending upon the details of the individual sterilization processes, their ability to defend their practices relating to load size variation may not be adequate. 蒸汽灭菌是许多制药和医疗器械产品制造中的关键过程。由于其重要性和广泛使用,它受到了从业者和监管者的极大关注。尽管如此,与负载大小相关的某些方面却令人头疼,并可能在灭菌循环开发和验证过程中造成困难。监管者通常会在预批准和常规检查中询问用户他们如何验证不同负载大小。监管期望是用户已经验证了固定的负载模式,因此所有灭菌过程的负载大小都应经过验证。 这适用于部分(多孔)和终端(非多孔)负载。尽管一些公司使用了定义的负载,但它们的使用限制了操作灵活性,许多工业用户寻求更灵活、更有效的方法。根据个别灭菌过程的细节,他们对负载大小变化的实践的辩护可能不够充分。 It has been common practice to utilize maximum loads as "worst case" demonstrations of sterilization lethality and assume that smaller size loads will be appropriately sterilized. The assumption is that the larger size, number, and mass of the maximum load represents a worst case and challenges the sterilization process sufficiently during validation that the minimum load (and all intermediate size loads) can be considered acceptable by default. Depending upon the manner in which the sterilizer is controlled during the process, this may or may not be true. Varying air removal, and/or differences in heating /cooling rate as is possible with varying load size, can result in process differences that can result in reduced cycle efficacy. 通常的做法是利用最大载荷作为灭菌致死的“最坏情况”证明,并假设较小尺寸的载荷将被适当灭菌。假设是,最大负载的大小、数量和质量代表了最坏情况,并在验证过程中充分挑战了灭菌过程,使得最小负载(以及所有中间大小的负载)默认可以接受。根据灭菌器在过程中的控制方式,这可能是或可能不是真的。不同的空气去除和/或加热/冷却速率的差异(如负载大小的变化)可能导致过程差异,从而降低循环效力。  This article will address the effect of load size on both parts (porous load) and terminal sterilization processes. The specific means to address load size differ between the sterilization processes; however the general approach to follow is similar. Using the content provided herein, the practitioner can establish their sterilization processes for either porous or non-porous loads with confidence that they can meet regulatory expectations, while maximizing operational flexibility. 本文将讨论负载大小对部件(多孔负载)和终端灭菌过程的影响。解决负载大小问题的具体方法因灭菌过程而异;然而,一般而言方法是相似的。使用本文提供的内容,从业者可以自信的为多孔或无孔负载建立灭菌循环,以满足监管期望,同时最大化操作灵活性。 1、PARTS (POROUS) CYCLES 部件(多孔)循环
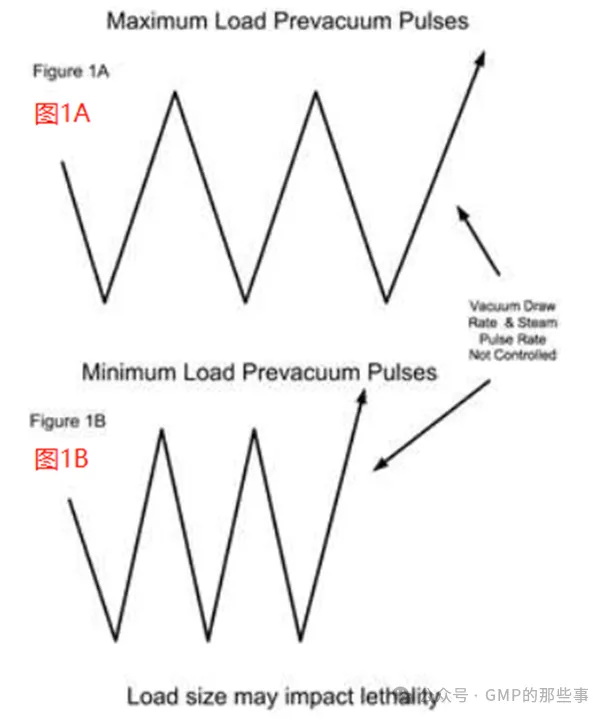
The maximum load should be studied initially in the validation effort. The maximum load can be an actual load, or one that is artificially created for the validation exercise. Depending upon the sterilizer usage there may be several different maximum loads. The maximum load(s) should be well documented as to component load item size, number and mass. The load items should have been previously evaluated to determine the "slowest to heat" location when properly wrapped and oriented. This should be performed with temperature sensors introduced in a way that does not compromise the integrity of the wrapping materials. The maximum load(s) are then subjected to validation in replicate studies with thermocouples and biological indicators demonstrating that sufficient lethality has been delivered throughout the load. 在验证工作中,首先应该研究最大负载。最大负载可以是实际负载,或者是为验证而人为创建的负载。根据灭菌器的使用情况,可能会有几种不同的最大负载。应详细记录最大负载的组件大小、数量和质量。负载项应事先评估,以确定在正确包装和定位时的“最难加热”的位置。这应该以一种不破坏包装材料完整性的方式引入温度传感器来完成。然后,通过热电偶和生物指示器对最大负载进行多次验证,以证明整个负载已传递足够的致死性。 A major consideration with porous loads is that air must be efficiently removed from the load. As the maximum load typically has many wrapped items, the rate at which air can be removed can be influenced by the load size. Air present within wrapped items must diffuse through the wrapping, whereas air removal from outside the load has no restrictions and is rapidly removed (see Figure 1A). When a minimum load is processed, the amount of internal air is reduced and it is possible that air within the load items may not be adequately removed when the targeted vacuum level is attained (see Figure 1B). The presence of air retained within the load item can result in the minimum load receiving less lethality than necessary for its reliable sterilization. The means to address this potential problem vary with the sterilizer control system and its sophistication. Note that in all of the scenarios the number of vacuum pulses would be the same and defined by what is necessary to meet any defined equilibration time requirements. 多孔负载的一个主要考虑因素是必须有效地从负载中除去空气。由于最大负载通常有许多包裹物品,因此可以去除空气的速率可能受到负载大小的影响。包裹物品内部的空气必须通过包裹扩散,而从负载外部排出的空气没有限制,并且会迅速排出(见图1A)。当处理最小负载时,内部空气量减少,当达到目标真空水平时,负载项目内的空气可能无法充分排出(见图1B)。负载项目中保留的空气的存在可能导致最小负载接收的杀伤力小于其可靠灭菌所需的杀伤力。解决这一潜在问题的方法因灭菌器控制系统及其复杂程度而异。请注意,在所有的情况下,真空脉冲的数量将是相同的,并由满足任何定义的平衡时间要求所定义的。 1.1、Steam sterilizer with pressure setpoints, vacuum and steam injection rate control 具有压力设定点、真空和蒸汽注入速率控制的蒸汽灭菌器
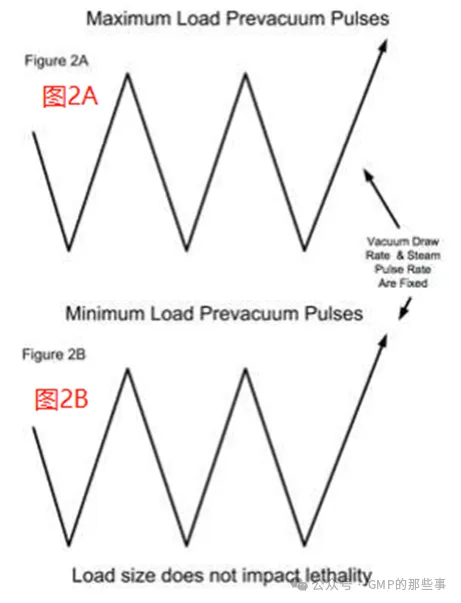
A sterilizer with this capability allows the rate of chamber pressure drawdown and steam injection rate during the vacuum pulses to be controlled. This is the optimal design, as controlled rates can be established such that the impact of load size is eliminated. Once confirmation of adequate air removal is established with the maximum load and verified with a minimum load the same vacuum draw down rate and steam injection rate are utilized used for all loads in the sterilizer regardless of size. The ramp rates should be set at no greater than 90% of the maximum possible for more reliable control. The pre-vacuum and steam pulse duration for the sterilizer will thus be reproducible allowing for complete air removal regardless of load size. The overall sterilization cycle times for all load sizes should be nearly identical (see Figures 2A and 2B). With this control approach, validation of the maximum load(s) would support smaller loads comprised of similar items. To ease regulatory questions, a single challenge run for the minimum with thermocouples and biological indicators should be performed. 具有这种能力的灭菌器允许在真空脉冲期间控制室压下降率和蒸汽注入率。这是最优设计,因为可以建立控制速率,从而消除负载大小的影响。一旦在最大负荷下确定了足够的空气排出量,并在最小负荷下确认了足够的空气排出量,那么对于灭菌器中的所有负荷,无论大小,都使用相同的真空抽吸率和蒸汽注入率。斜坡速率应设置为不大于最大可能值的90%,以获得更可靠的控制。因此,灭菌器的预真空和蒸汽脉冲持续时间将是可重复的,无论负载大小如何,都可以完全去除空气。所有负载大小的总体灭菌周期时间应该几乎相同(参见图2A和2B)。使用这种控制方法,最大负载的验证将支持由类似项目组成的较小负载。为了缓解监管问题,应使用热电偶和生物指示剂进行最低限度的单次挑战。 1.2、Sterilizer with pressure set points and vacuum/pressure hold time control 具有压力设定点和真空/压力保持时间控制的灭菌器
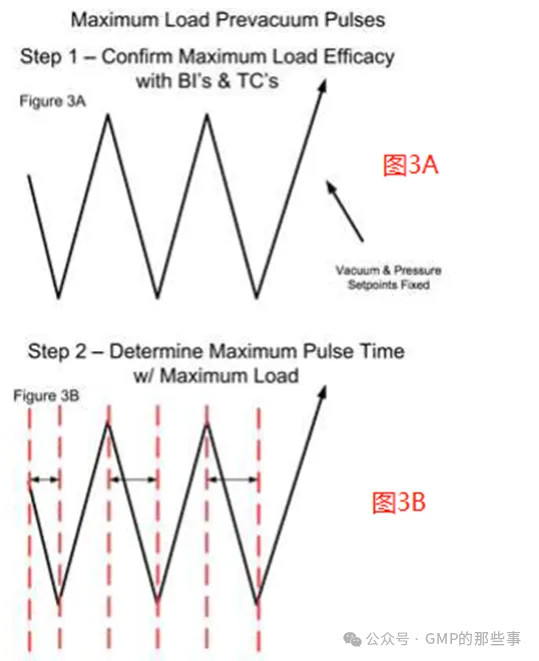
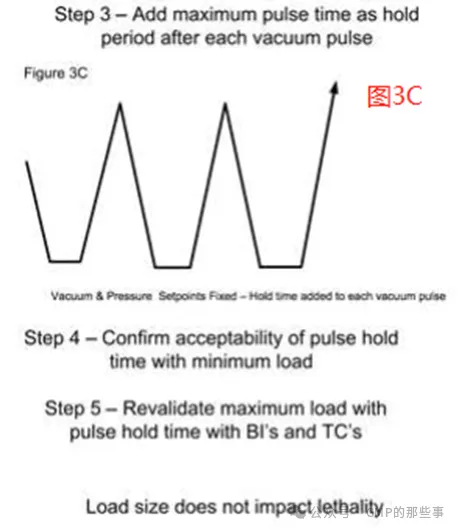
With this type of control, the vacuum drawdown time can vary with load size, with maximum loads typically requiring the longest time to attain the vacuum setpoint. The maximum load validation would be performed with no added hold period in each vacuum pulse and proven acceptable with physical and biological data (see Figures 3A and 3B). Next, the time required to draw the vacuum to setpoint of the maximum load would be established as the hold time at full vacuum for each vacuum pulse (see Figure 3C). Even though the steam pressurization rate may be less important, a similar increment of hold time at the end of each steam injection is also used. This approach increases the full duration of the vacuum drawdown and steam pulses to that required for the maximum load, and assure that the minimum load has adequate time for air removal/steam penetration. The overall cycle time is extended only slightly because of the added hold times after each vacuum/steam pulse. The overall cycle times with this control scheme would be slightly longer than what is possible with vacuum/steam injection rate control. Nevertheless, even this less refined sterilizer control, validation of the maximum load(s) supports the sterilization of smaller loads comprised of similar items. A single run minimum load with thermocouples and biological indicators should be performed to confirm the process. 使用这种类型的控制,真空下降时间可以随负载大小而变化,最大负载通常需要最长的时间才能达到真空设定值。最大负荷验证将在每个真空脉冲不增加保持时间的情况下进行,并通过物理和生物数据证明是可接受的(见图3A和3B)。接下来,将达到最大负载真空设定值所需的时间作为每个真空脉冲在全真空状态下的保持时间(见图3C)。尽管蒸汽增压率可能不太重要,但在每次注汽结束时也使用类似的保持时间增量。这种方法将真空下降和蒸汽脉冲的持续时间增加到最大负载所需的时间,并确保最小负载有足够的时间进行空气排出/蒸汽渗透。由于每次真空/蒸汽脉冲后增加的保持时间,整个循环时间仅略微延长。这种控制方案的总循环时间将比真空/蒸汽注入速率控制的周期略长。然而,即使这种不太精细的灭菌器控制,最大负载的验证也支持由类似物品组成的较小负载的灭菌。应使用热电偶和生物指示剂进行单次最小负荷运行,以确认该过程。
1.3、Sterilizer with vacuum/pressure set points only 仅有真空/压力设定点的灭菌器 Because neither the vacuum drawdown rate nor the vacuum hold time is controlled, this type of sterilizer control system is not amenable to the methods described above to assure adequate sterilization of minimum loads (see Figures iA and iB). Validation of this sterilizer design requires consideration of minimum and maximum loads in triplicate studies using thermocouples and biological indicators. 由于真空降速和真空保持时间都不受控制,这种类型的灭菌器控制系统不适合上述方法,以确保对最小负荷进行充分的灭菌(见图1A和图1B)。该灭菌器设计的验证需要在使用热电偶和生物指示剂的三次重复研究中考虑最小和最大负荷。 1.4、TERMINAL STERILIZATION CYCLES 终端灭菌周期 The sterilization of liquid filled aqueous containers represents a more challenging situation in that not only is there a minimum lethality expectation; the materials in the load must also not be over-processed. The means to assure consistency across load size is often facilitated because terminal sterilizers are ordinarily equipped with more sophisticated control systems allowing for the regulation of heating and cooling rates. Before commencing the effort, the lethality required to sterilize the filled containers must be chosen from container mapping studies and information on the expected bioburden resistance and population. This calculation will typically include some added margin of safety to ease routine operation. 充满液体的含水容器的灭菌是一种更具挑战性的情况,因为不仅有最低的死亡率预期;负载中的材料也不能过度加工。由于终端灭菌器通常配备了更复杂的控制系统,允许调节加热和冷却速率,因此确保负载大小一致性的手段通常很容易。在开始工作之前,必须从容器温度分布研究和有关预期生物负荷抗性和种群的信息中选择对灌装容器进行灭菌所需的致死率。这种计算通常包括一些额外的安全余量,以简化日常操作。 Replicate mapping studies with the maximum load are performed next to identify the minimum and maximum lethality locations (these are more commonly a region or zone than an individual container). During these studies the heating and cooling rates should be set at approximately 90% of the equipment's full capability (see Figure 4A). This value is selected to ensure controllability while not extending the overall cycle appreciably. 在最大载荷下进行重复温度分布研究,以确定最小和最大致死位置(这些位置通常是一个区域,而不是单个容器)。在这些研究中,加热和冷却速率应设置在设备全部能力的90%左右(见图4A)。选择此值是为了确保可控性,同时不会明显延长整个周期。
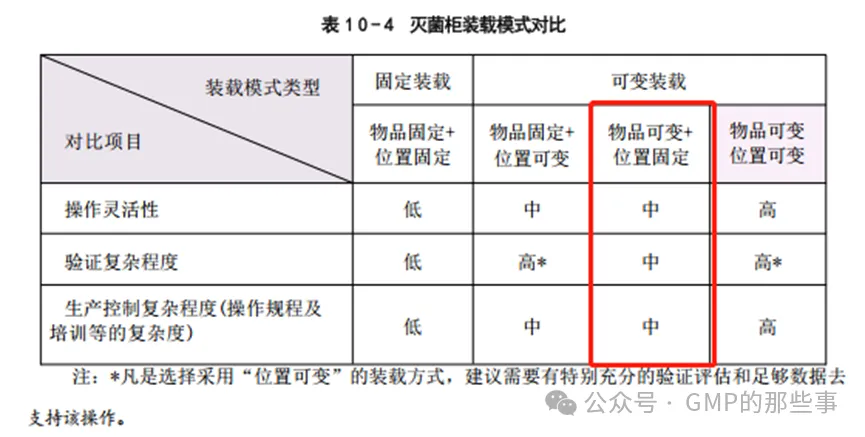
The critical concern in terminal sterilization is that the amount of heat to sterilize the filled containers varies with load size. The appropriate process dwell for the cycle must be established by adjusting for any lag time between the sterilization dwell control location (assumed to be somewhere in the chamber) and the 'slowest to heat' zone within the load. The lag time is greatest for the maximum load due to its greater mass but will vary based on load size (and to some extent upon the container size/product heat capacity as well). Were the chamber heating/cooling rates allowed to vary freely for different load sizes, the dwell period, which is usually established from a probe outside the load, may start prematurely and sufficient lethality might not be delivered to smaller loads (see Figure 4B). A similar situation occurs during cooling of the load and has a similar effect.The smaller the load, the less affect it has on the overall heating of the chamber and, thus, on the initiation of the dwellperiod. However, it should not be assumed that the parameters used for the maximum load are directly applicable to the minimum load. Both extremes should be confirmed, with the lag between chamber and load temperatures being determined experimentally. Because terminal sterilization processes must also consider the maximum conditions for their impact of product quality attributes, the comparative evaluation must consider high Fo locations as well, If the maximum and minimum lethality ranges are equivalent the control strategy is acceptable. Validation studies would then be performed for both maximum and minimum loads, in triplicate, using temperature probes and bioindicators. Confirmation of sterilization performance for intermediate load sizes would not be required as the extreme load sizes have been proven successful using an identical sterilization process. 终端灭菌的关键问题是灭菌所装容器的热量随负载大小而变化。必须通过调整灭菌驻留控制位置(假设在腔室中的某个地方)和负载内“最慢加热”区域之间的任何滞后时间来建立适当的循环过程驻留。最大负载的滞后时间最大,因为它的质量更大,但会根据负载大小而变化(在某种程度上也取决于容器尺寸/产品热容)。如果允许不同负载大小的腔室加热/冷却速率自由变化,则通常由负载外的探头确定的停留期(灭菌阶段)可能过早开始,并且可能无法向较小的负载传递足够的致命性(见图4B)。在负载冷却过程中会发生类似的情况,并且具有类似的影响。负载越小,它对腔室整体加热的影响就越小,因此,对开始保温期的启动影响也就越小。但是,不应假定用于最大载荷的参数直接适用于最小载荷。这两个极端都应该得到确认,并通过实验确定腔室和负载温度之间的滞后。由于终端灭菌过程还必须考虑其对产品质量属性影响的最大条件,因此比较评价也必须考虑高F0位置,如果最大和最小致死范围相等,则控制策略是可接受的。然后使用温度探针和生物指示剂对最大和最小负荷进行验证研究,一式三份。不需要确认中等负载尺寸的灭菌性能,因为极端负载尺寸已被证明使用相同的灭菌过程是成功的。 Conclusion结论 The methods outlined should enable the practitioner to efficiently and confidently validate maximum and minimum loads undergoing steam sterilization with a fuller understanding of the control elements that will assure consistency of efficacy, The control suggestions indicated allow for greater flexibility in operation while maintaining the required lethality and product stability which are essential to regulatory compliance. 概述的方法应使从业者能够高效且自信地验证蒸汽灭菌过程中的最大和最小负载,更全面地理解将确保效力一致性的控制要素。所指示的控制建议允许在保持所需的致死性和产品稳定性的同时,具有更大的操作灵活性,这对于监管合规性至关重要。 以下内容源于GMP实施指南2023-无菌制剂上册 湿热灭菌常见问题讨论 问题1:湿热灭菌柜的装载模式有哪些?对于非产品的固体物品(多孔/坚硬类的物品, 如工器具、无菌服等)的灭菌,是否可采用“ 可变装载” 的方式?需考虑哪些因素? 解答: 通常情况下,灭菌柜的固体物品装载模式可有如下几种情况: ● 固定装载:物品固定+位置固定。 ●可变装载: o 物品固定+位置可变。 o 物品可变+位置固定。 o 物品可变+位置可变。 物品是指在灭菌柜中所有的需灭菌物品, 位置指的是单个灭菌物品在灭菌柜中的放置位置。 例如“ 物品可变” 即为灭菌物品的数量, 可在最小装载和最大装载的范围进行变化; “位置可变” 即为灭菌物品的位置可在灭菌柜中变化, 如放置在灭菌推车的任意一层。 对于可变装载而言,用的比较多的是“物品可变+位置固定”
对于使用“物品可变” 的可变装载模式,需描述最大装载和最小装载形式。最大装载一般可采用实际需求的最大物品量,最小装载物品可基于物品灭菌的热力表现进行选择, 一般可选择最具有灭菌挑战的物品,例如可考虑选择最低的F0数值等表征灭菌效果的数据做为最小装载物品选择的依据。 通常需对最大装载和最小装载进行开发和验证。 对于使用“位置可变” 的可变装载模式,前提是温度分布研究可以证明灭菌柜腔体内在程序灭菌阶段温度的均一性,并且通过开发和验证可以确认:当物品放置在不同位置时, 平衡时间、 F0值、 干燥效果等关键指标能够满足标准的要求。 通常也需对最大装载和最小装载进行开发和验证。 上述描述适用于固体装载采用脉动真空工艺的灭菌方式, 对于液体装载或产品装载的湿热灭菌开发和验证的策略,重点是要评估出“最差条件”的情况,可基于如产品特性、容器规格和装量等进行分组考虑, 需经过科学合理的评估制定装载模式的开发和验证的策略。 —————————————————— 为人类感控事业而奋斗——明誉医疗 |